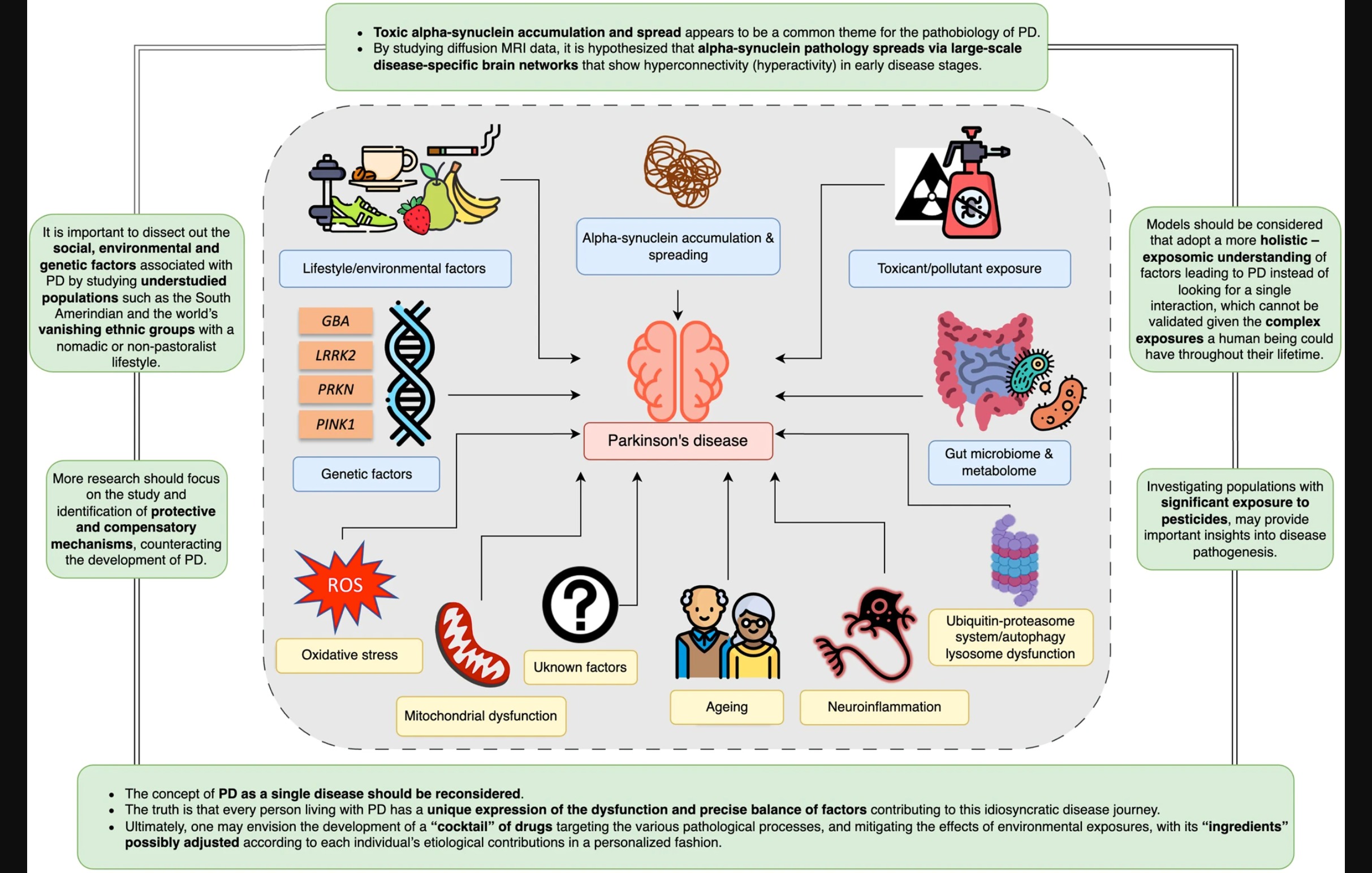
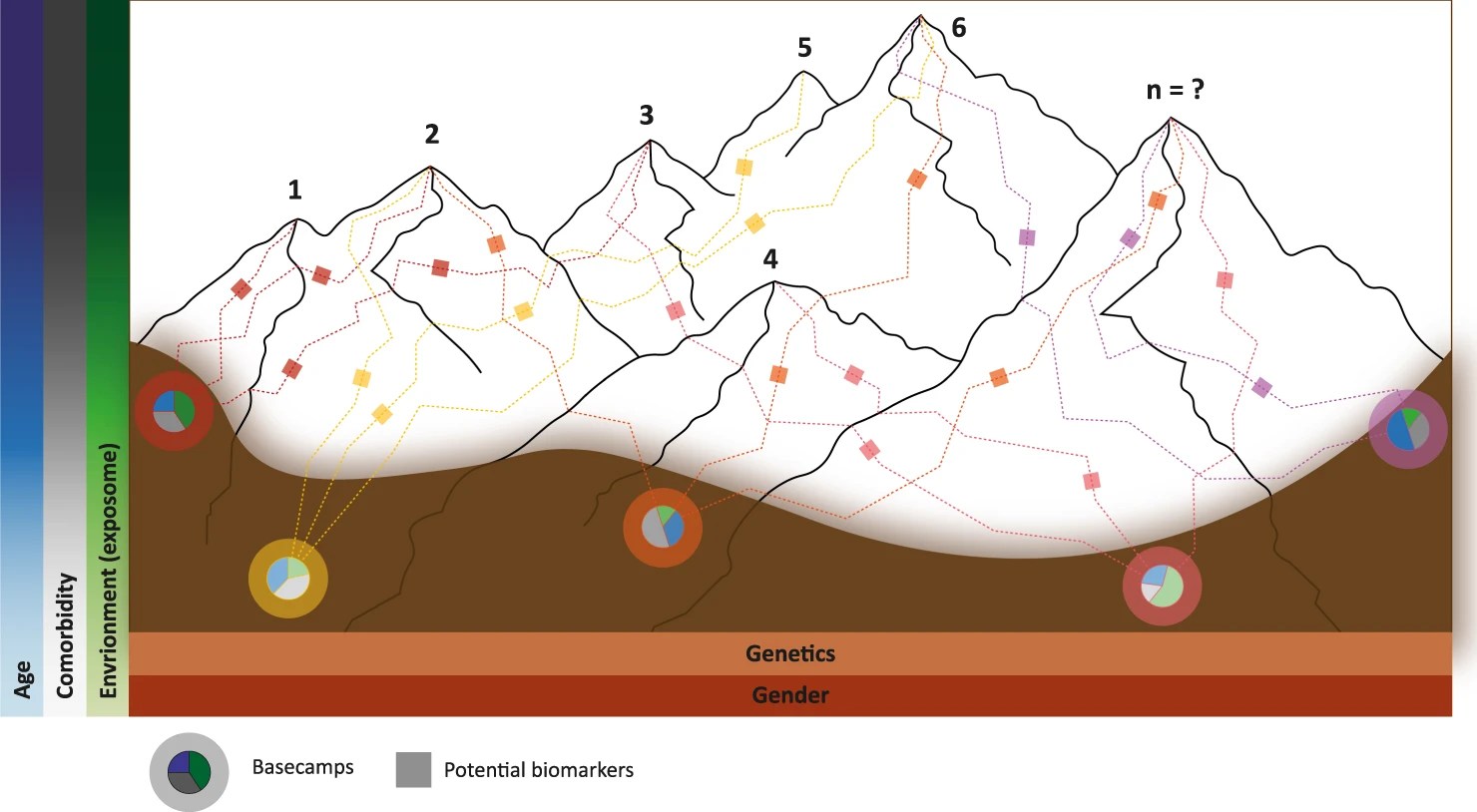
As a person who has had essential tremors ever since his teenage years, and a person who was diagnosed with Parkinson’s disease at the average onset age of 62 years, it was with great interest that the author reviewed the abstracts and full text open access PubMedCenter articles available on the topic.
The author can recall others (medical professionals included) who did not observe tremors, although this subject could feel the tremors within, during teenage years. During undergraduate studies, he once was told that his initial impression on a fellow college student looking to sublet a room was that of a “speed freak” (methamphetamine abuser to those unfamiliar with the term). At age 28, his future wife remarked on his trembling hands as he reached out to touch her cheek in a moment of intimacy. And at age 60, following several recurring episodes of depression and increasing tremors, he was treated for essential tremors for almost two years, beginning in 2010. Then, after having opted for Deep Brain Stimulation (DBS), and undergoing further testing for several months, a third symptom, rigidity, one of the four cardinal symptoms of PD, was observed, allowing the Movement Disorder Specialist to finally make a diagnosis of PD. Mind you, the four cardinal symptoms required to make the call haven’t changed since 1817, when James Parkinson published his Essay On The Shaking Palsy. In case you don’t already know, they are Tremors, Postural Instability, Bradykinesia, and Rigidity.
On the other hand, it was only in 2009 that the study “LINGO1 rs9652490 is associated with essential tremor and Parkinson disease” came out online and published in print a few months later in 2010. In which year, the observant reader will notice, was when this author began receiving treatments for essential tremors, finally diagnosed in late 2011 as Parkinson’s.
After a few articles reported similar results, a few negative results were reported, in part by one group of desk jockeys who conducted a meta-review and concluded that there was NO association between ET and PD. On looking just briefly at the abstracts, one can see that those that found a positive connection and those that found no connection were looking at different populations. The positive association came from a European background, and those with no association came from a Chinese population sample. Mix them all together and you get mixed results, which doesn’t mean that a person with a half European background (like myself) with this specific variation doesn’t have an increased risk of developing ET (P = 0.014) and Parkinson’s(P = 0.0003), as reported in the article noted above.
So who should I believe? A study that involved a sample in which the participants were similar in ethnic/DNA background to mine? Or a meta-review which concludes that the null hypothesis is true? Or my own brain and body, which began exhibiting strong tremors back in late teens to early twenties? And which became so severe by age 60 that I couldn’t carry a cup of coffee from the kitchen to the living room without going into extreme oscillations?
Those were rhetorical questions, by the way.
References:
Jiménez-Jiménez FJ, García-Martín E, Lorenzo-Betancor O, Pastor P, Alonso-Navarro H, Agúndez JA. LINGO1 and risk for essential tremor: results of a meta-analysis of rs9652490 and rs11856808. J Neurol Sci. 2012 Jun 15;317(1-2):52-7. doi: 10.1016/j.jns.2012.02.030. Epub 2012 Mar 17. PMID: 22425540.
Agúndez JA, Lorenzo-Betancor O, Pastor P, García-Martín E, Luengo A, Alonso-Navarro H, Jiménez-Jiménez FJ. LINGO1 rs9652490 and rs11856808 are not associated with the risk of Parkinson’s disease: results of a meta-analysis. Parkinsonism Relat Disord. 2012 Jun;18(5):657-9. doi: 10.1016/j.parkreldis.2011.09.005. Epub 2011 Sep 28. PMID: 21955595.
Lorenzo-Betancor O, Samaranch L, García-Martín E, Cervantes S, Agúndez JA, Jiménez-Jiménez FJ, Alonso-Navarro H, Luengo A, Coria F, Lorenzo E, Irigoyen J, Pastor P; Iberian Parkinson’s Disease Genetics Study Group Researchers. LINGO1 gene analysis in Parkinson’s disease phenotypes. Mov Disord. 2011 Mar;26(4):722-7. doi: 10.1002/mds.23452. Epub 2011 Jan 4. PMID: 21506150.
Wu YW, Rong TY, Li HH, Xiao Q, Fei QZ, Tan EK, Ding JQ, Chen SD. Analysis of Lingo1 variant in sporadic and familial essential tremor among Asians. Acta Neurol Scand. 2011 Oct;124(4):264-8. doi: 10.1111/j.1600-0404.2010.01466.x. Epub 2010 Dec 15. PMID: 21158743.
Zuo X, Jiang H, Guo JF, Yu RH, Sun QY, Hu L, Wang L, Yao LY, Shen L, Pan Q, Yan XX, Xia K, Tang BS. Screening for two SNPs of LINGO1 gene in patients with essential tremor or sporadic Parkinson’s disease in Chinese population. Neurosci Lett. 2010 Sep 6;481(2):69-72. doi: 10.1016/j.neulet.2010.06.041. Epub 2010 Jun 19. PMID: 20600614.
Clark LN, Park N, Kisselev S, Rios E, Lee JH, Louis ED. Replication of the LINGO1 gene association with essential tremor in a North American population. Eur J Hum Genet. 2010 Jul;18(7):838-43. doi: 10.1038/ejhg.2010.27. Epub 2010 Apr 7. PMID: 20372186; PMCID: PMC2987362.
Nica AC, Montgomery SB, Dimas AS, Stranger BE, Beazley C, Barroso I, Dermitzakis ET. Candidate causal regulatory effects by integration of expression QTLs with complex trait genetic associations. PLoS Genet. 2010 Apr 1;6(4):e1000895. doi: 10.1371/journal.pgen.1000895. PMID: 20369022; PMCID: PMC2848550.
Vilariño-Güell C, Wider C, Ross OA, Jasinska-Myga B, Kachergus J, Cobb SA, Soto-Ortolaza AI, Behrouz B, Heckman MG, Diehl NN, Testa CM, Wszolek ZK, Uitti RJ, Jankovic J, Louis ED, Clark LN, Rajput A, Farrer MJ. LINGO1 and LINGO2 variants are associated with essential tremor and Parkinson disease. Neurogenetics. 2010 Oct;11(4):401-8. doi: 10.1007/s10048-010-0241-x. Epub 2010 Apr 6. PMID: 20369371; PMCID: PMC3930084.
Wu Y, Wang X, Xu W, Liu W, Fang F, Ding J, Song Y, Chen S. Genetic variation in LINGO-1 (rs9652490) and risk of Parkinson’s disease: twelve studies and a meta-analysis. Neurosci Lett. 2012 Jul 26;522(1):67-72. doi: 10.1016/j.neulet.2012.06.018. Epub 2012 Jun 15. PMID: 22710005.
Jasinska-Myga B, Wider C. Genetics of essential tremor. Parkinsonism Relat Disord. 2012 Jan;18 Suppl 1:S138-9. doi: 10.1016/S1353-8020(11)70043-8. PMID: 22166413.
Bourassa CV, Rivière JB, Dion PA, Bernard G, Diab S, Panisset M, Chouinard S, Dupré N, Fournier H, Raelson J, Belouchi M, Rouleau GA. LINGO1 variants in the French-Canadian population. PLoS One. 2011 Jan 11;6(1):e16254. doi: 10.1371/journal.pone.0016254. PMID: 21264305; PMCID: PMC3019170.
Guo Y, Jankovic J, Song Z, Yang H, Zheng W, Le W, Tang X, Deng X, Yang Y, Deng S, Luo Z, Deng H. LINGO1 rs9652490 variant in Parkinson disease patients. Neurosci Lett. 2011 Jan 7;487(2):174-6. doi: 10.1016/j.neulet.2010.10.016. Epub 2010 Oct 15. PMID: 20951767.
Thier S, Lorenz D, Nothnagel M, Stevanin G, Dürr A, Nebel A, Schreiber S, Kuhlenbäumer G, Deuschl G, Klebe S. LINGO1 polymorphisms are associated with essential tremor in Europeans. Mov Disord. 2010 Apr 30;25(6):717-23. doi: 10.1002/mds.22887. PMID: 20310002.
Vilariño-Güell C, Ross OA, Wider C, Jasinska-Myga B, Cobb SA, Soto-Ortolaza AI, Kachergus JM, Keeling BH, Dachsel JC, Melrose HL, Behrouz B, Wszolek ZK, Uitti RJ, Aasly JO, Rajput A, Farrer MJ. LINGO1 rs9652490 is associated with essential tremor and Parkinson disease. Parkinsonism Relat Disord. 2010 Feb;16(2):109-11. doi: 10.1016/j.parkreldis.2009.08.006. Epub 2009 Aug 31. PMID: 19720553; PMCID: PMC2844122.
Deng H, Gu S, Jankovic J. LINGO1 variants in essential tremor and Parkinson’s disease. Acta Neurol Scand. 2012 Jan;125(1):1-7. doi: 10.1111/j.1600-0404.2011.01516.x. Epub 2011 Apr 7. PMID: 21470193.
Zimprich A. Genetics of Parkinson’s disease and essential tremor. Curr Opin Neurol. 2011 Aug;24(4):318-23. doi: 10.1097/WCO.0b013e3283484b87. PMID: 21734494.